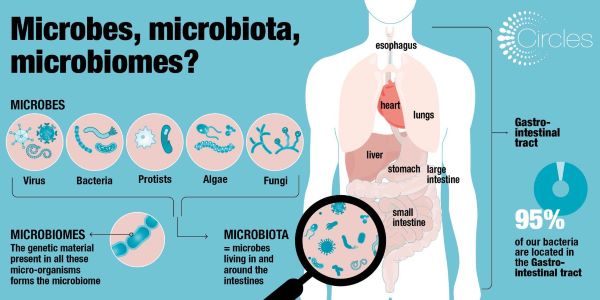
Infographic: Microbes, Microbiota & Microbiomes




From BMJ Journal “Gut” study (February 17, 2020):
 We observed that increased adherence to the MedDiet modulates specific components of the gut microbiota that were associated with a reduction in risk of frailty, improved cognitive function and reduced inflammatory status.
We observed that increased adherence to the MedDiet modulates specific components of the gut microbiota that were associated with a reduction in risk of frailty, improved cognitive function and reduced inflammatory status.
Objective Ageing is accompanied by deterioration of multiple bodily functions and inflammation, which collectively contribute to frailty. We and others have shown that frailty co-varies with alterations in the gut microbiota in a manner accelerated by consumption of a restricted diversity diet. The Mediterranean diet (MedDiet) is associated with health. In the NU-AGE project, we investigated if a 1-year MedDiet intervention could alter the gut microbiota and reduce frailty.
Design We profiled the gut microbiota in 612 non-frail or pre-frail subjects across five European countries (UK, France, Netherlands, Italy and Poland) before and after the administration of a 12-month long MedDiet intervention tailored to elderly subjects (NU-AGE diet).
Results Adherence to the diet was associated with specific microbiome alterations. Taxa enriched by adherence to the diet were positively associated with several markers of lower frailty and improved cognitive function, and negatively associated with inflammatory markers including C-reactive protein and interleukin-17. Analysis of the inferred microbial metabolite profiles indicated that the diet-modulated microbiome change was associated with an increase in short/branch chained fatty acid production and lower production of secondary bile acids, p-cresols, ethanol and carbon dioxide. Microbiome ecosystem network analysis showed that the bacterial taxa that responded positively to the MedDiet intervention occupy keystone interaction positions, whereas frailty-associated taxa are peripheral in the networks.
Conclusion Collectively, our findings support the feasibility of improving the habitual diet to modulate the gut microbiota which in turn has the potential to promote healthier ageing.
Most of the time your digestive tract toils silently in the background, routinely taking in nourishment and expelling waste. But here’s a key takeaway: Your brain is a critical part of maintaining this smoothly running system.
Here to explain is Harvard professor Dr. Lawrence S. Friedman, faculty editor the special health report Sensitive Gut.
Raja Dhir is the co-founder of microbiome company Seed. Based in LA, Seed is a collective of scientists and doctors, researching how bacteria can improve human health and that of our planet. Its first product, a daily synbiotic, focuses on the stomach.
Raja Dhir is a life sciences entrepreneur and Co-Founder of Seed, a venture-backed  microbiome company pioneering the application of bacteria for both human and planetary health. He leads Seed’s R&D, academic collaborations, technology development, clinical trial design, supply chain, and intellectual property strategy.
microbiome company pioneering the application of bacteria for both human and planetary health. He leads Seed’s R&D, academic collaborations, technology development, clinical trial design, supply chain, and intellectual property strategy.
 Together with Dr. Jacques Ravel, he Co-Chairs Seed’s Scientific Advisory Board–an interdisciplinary group of scientists and doctors who lead research teams and teach at institutions including the teaching hospital of Harvard Medical School and the Trial Innovation Unit of Mass. General Hospital (MGH). Raja has designed clinical trials with leading academic institutions including the teaching hospital of Harvard Medical School and the Trial Innovation Unit of Mass. General Hospital (MGH).
Together with Dr. Jacques Ravel, he Co-Chairs Seed’s Scientific Advisory Board–an interdisciplinary group of scientists and doctors who lead research teams and teach at institutions including the teaching hospital of Harvard Medical School and the Trial Innovation Unit of Mass. General Hospital (MGH). Raja has designed clinical trials with leading academic institutions including the teaching hospital of Harvard Medical School and the Trial Innovation Unit of Mass. General Hospital (MGH).
Raja has unique expertise translating scientific research for product development with a track record that includes patented inventions to stabilize sensitive compounds to improve alpha-diversity of the gut microbiome (derived from micro-algae) and most recently, the co-invention of microbial technologies to protect honeybee populations (Apis mellifera) from neonicotinoid pesticides and pathogen colonization. His work also includes biofermentation and scale-up for both facultative and strict anaerobic organisms.
From a Science Magazine online article:
Antibiotic use diminished the gut microbiome composition and impaired the ability of the immune system to generate antibodies. Treatment with antibiotics also disturbed bile acid metabolism and caused inflammatory responses.
From the original findings the Journal “Cell.com”:
Emerging evidence indicates a central role for the microbiome in immunity. However, causal evidence in humans is sparse. Here, we administered broad-spectrum antibiotics to healthy adults prior and subsequent to seasonal influenza vaccination. Despite a 10,000-fold reduction in gut bacterial load and long-lasting diminution in bacterial diversity, antibody responses were not significantly affected. However, in a second trial of subjects with low pre-existing antibody titers, there was significant impairment in H1N1-specific neutralization and binding IgG1 and IgA responses.
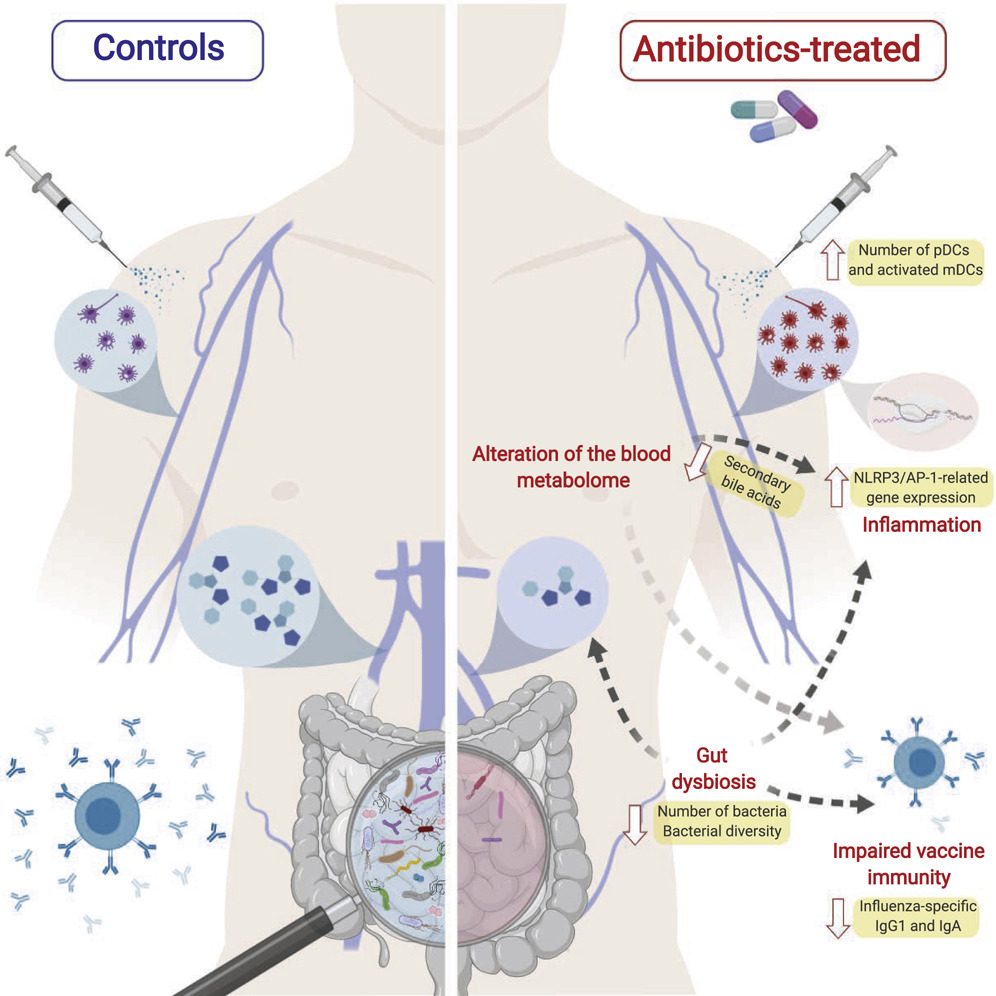
In addition, in both studies antibiotics treatment resulted in (1) enhanced inflammatory signatures (including AP-1/NR4A expression), observed previously in the elderly, and increased dendritic cell activation; (2) divergent metabolic trajectories, with a 1,000-fold reduction in serum secondary bile acids, which was highly correlated with AP-1/NR4A signaling and inflammasome activation. Multi-omics integration revealed significant associations between bacterial species and metabolic phenotypes, highlighting a key role for the microbiome in modulating human immunity.