Tag Archives: Health Update
Health: Wear “Cloth Face Mask” In Public To Prevent Spread Of Coronavirus
New research suggests that face masks may offer more protection against coronavirus infection than previously thought. It suggests that coughs and sneezes may be projected much further than scientists had thought possible. The World Health Organisation is considering whether to update its guidance on face masks and the White House may recommend that Americans wear them. Meanwhile in the UK hospitality companies are turning their skills to help those in need. And for the second week in a row, applause has rung out from members of the public showing gratitude to NHS staff and other workers helping to keep the country going. Sophie Raworth presents BBC News at Ten coverage from Science Editor David Shukman and Social Affairs Correspondent Alison Holt.
Coronavirus / Covid-19: “When Will We Have A Vaccine?” (Podcast)
 Scientists around the world are racing to develop a vaccine for COVID-19. But experts have said it could take a year to 18 months for one to hit the market. The process for testing and approving a vaccine is long and complicated.
Scientists around the world are racing to develop a vaccine for COVID-19. But experts have said it could take a year to 18 months for one to hit the market. The process for testing and approving a vaccine is long and complicated.
That can be frustrating when the coronavirus is taking more and more lives every day. But cutting corners to push a vaccine through faster can lead to devastating consequences. We know that, because it’s happened before.
Science: Coronavirus Pandemic Update And Nonanimal Testing Methods (Podcast)
 On this week’s show, host Joel Goldberg gets an update on the coronavirus pandemic from Senior Correspondent Jon Cohen. In addition, Cohen gives a rundown of his latest feature, which highlights the relationship between diseases and changing seasons—and how this relationship relates to a potential coronavirus vaccine.
On this week’s show, host Joel Goldberg gets an update on the coronavirus pandemic from Senior Correspondent Jon Cohen. In addition, Cohen gives a rundown of his latest feature, which highlights the relationship between diseases and changing seasons—and how this relationship relates to a potential coronavirus vaccine.
Also this week, from a recording made at this year’s AAAS annual meeting in Seattle, host Meagan Cantwell speaks with Alexandra Maertens, director of the Green Toxicology initiative at Johns Hopkins University, Baltimore, about the importance of incorporating nonanimal testing methods to study the adverse effects of chemicals.
Health Update: Ibuprofen Results In “More Severe Illness” For “Coronavirus / Covid-19” Patients (BMJ)
From a BMJ online release (March 17, 2020):
 “The finding in two randomised trials that advice to use ibuprofen results in more severe illness or complications helps confirm that the association seen in observational studies is indeed likely to be causal. Advice to use paracetamol (acetaminophen) is also less likely to result in complications.”
“The finding in two randomised trials that advice to use ibuprofen results in more severe illness or complications helps confirm that the association seen in observational studies is indeed likely to be causal. Advice to use paracetamol (acetaminophen) is also less likely to result in complications.”
Scientists and senior doctors have backed claims by France’s health minister that people showing symptoms of covid-19 should use paracetamol (acetaminophen) rather than ibuprofen, a drug they said might exacerbate the condition.
 Ian Jones, a professor of virology at the University of Reading, said that ibuprofen’s anti-inflammatory properties could “dampen down” the immune system, which could slow the recovery process. He added that it was likely, based on similarities between the new virus (SARS-CoV-2) and SARS I, that covid-19 reduces a key enzyme that part regulates the water and salt concentration in the blood and could contribute to the pneumonia seen in extreme cases. “Ibuprofen aggravates this, while paracetamol does not,” he said.
Ian Jones, a professor of virology at the University of Reading, said that ibuprofen’s anti-inflammatory properties could “dampen down” the immune system, which could slow the recovery process. He added that it was likely, based on similarities between the new virus (SARS-CoV-2) and SARS I, that covid-19 reduces a key enzyme that part regulates the water and salt concentration in the blood and could contribute to the pneumonia seen in extreme cases. “Ibuprofen aggravates this, while paracetamol does not,” he said.
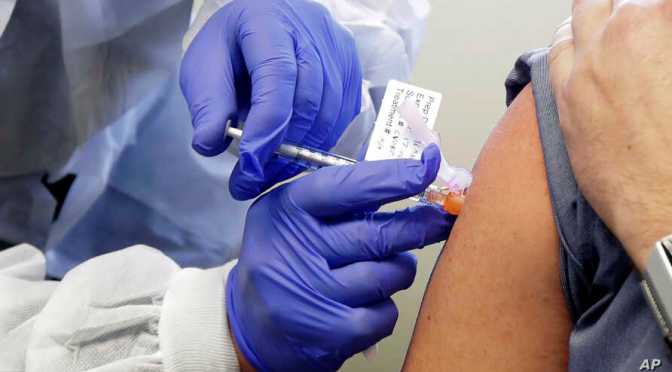
Health Update: The First Clinical Trial Of A “Coronavirus / Covid-19” Vaccine Begins (NIH)
From the National Institutes of Health (NIH) (March 17, 2020):
 “Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said NIAID Director Anthony S. Fauci, M.D. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said NIAID Director Anthony S. Fauci, M.D. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
The vaccine is called mRNA-1273 and was developed by NIAID scientists and their collaborators at the biotechnology company Moderna, Inc., based in Cambridge, Massachusetts. The Coalition for Epidemic Preparedness Innovations (CEPI) supported the manufacturing of the vaccine candidate for the Phase 1 clinical trial.
 A Phase 1 clinical trial evaluating an investigational vaccine designed to protect against coronavirus disease 2019 (COVID-19) has begun at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is funding the trial. KPWHRI is part of NIAID’s Infectious Diseases Clinical Research Consortium. The open-label trial will enroll 45 healthy adult volunteers ages 18 to 55 years over approximately 6 weeks. The first participant received the investigational vaccine today.
A Phase 1 clinical trial evaluating an investigational vaccine designed to protect against coronavirus disease 2019 (COVID-19) has begun at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is funding the trial. KPWHRI is part of NIAID’s Infectious Diseases Clinical Research Consortium. The open-label trial will enroll 45 healthy adult volunteers ages 18 to 55 years over approximately 6 weeks. The first participant received the investigational vaccine today.
The study is evaluating different doses of the experimental vaccine for safety and its ability to induce an immune response in participants. This is the first of multiple steps in the clinical trial process for evaluating the potential benefit of the vaccine.
Health Update: “New Coronavirus Test” From Johns Hopkins Medicine
New Coronavirus test developed by Johns Hopkins microbiologists Karen Carroll and Heba Mostafa, look to test as many as 1,000 suspected Covid-19 cases per day in the Johns Hopkins Health System.