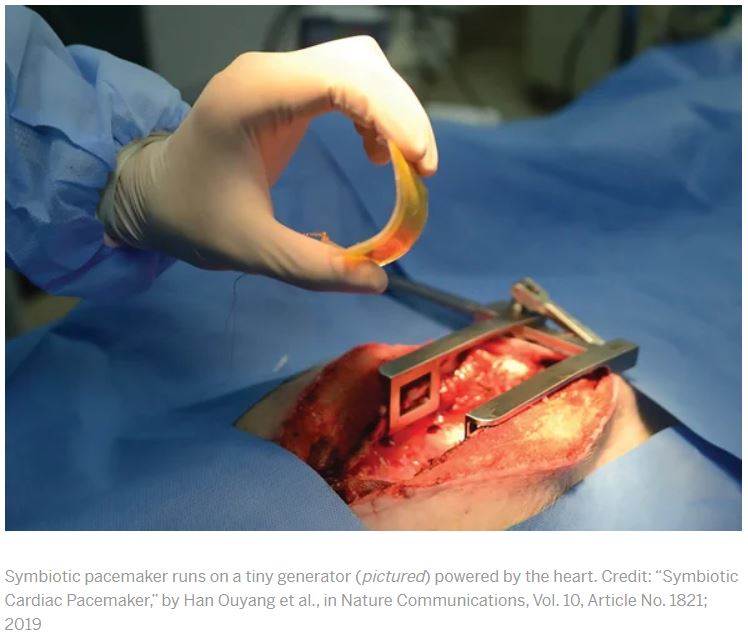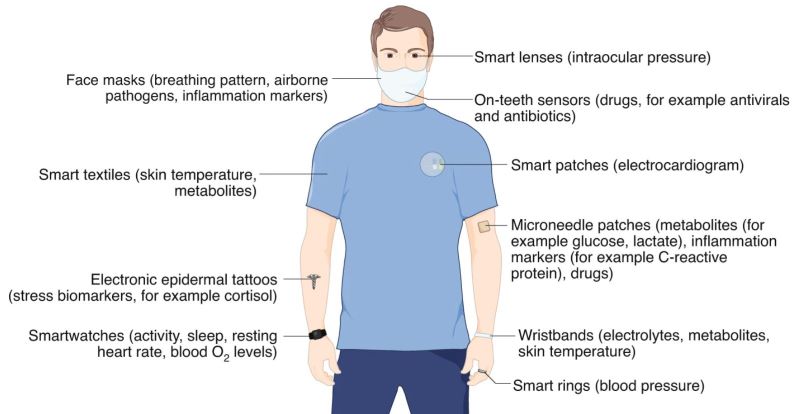

Currently, smartwatches provide information such as heart rate, sleep time and activity patterns. In the future, this could be augmented with new classes of wearable devices that monitor, for example, concentrations of cortisol for tracking stress (using electronic epidermal tattoos), biomarkers of inflammation and levels of blood O2 (microneedle patches), skin temperature (electronic textiles), blood pressure (smart rings), concentration of ions (wristbands), intraocular pressure (smart contact lenses), the presence of airborne pathogens and breathing anomalies (face masks), and the concentration of therapeutic drugs (on-teeth sensors)2,10,12,13,14,15,16. Such emerging low-cost wearable sensing technologies, monitoring both physical parameters and biochemical markers, could be used to identify symptomatic and pre-symptomatic cases in future pandemics. The devices could also be used to remotely monitor the recovery of individuals undergoing treatment or self-isolating at home.

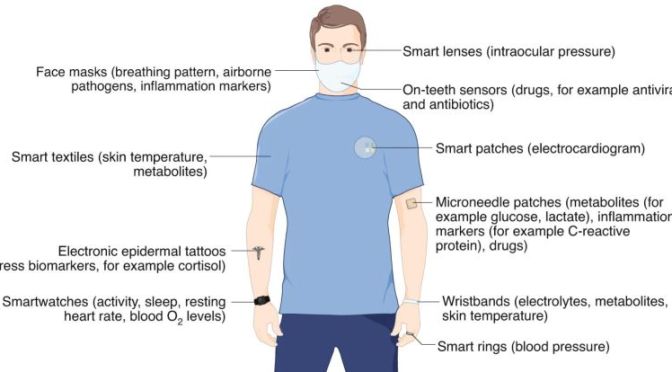

 cardiovascular events.
cardiovascular events.