The worsening pandemic continues to mean millions of Americans out of work. For older employees in particular, this kind of long-term unemployment can represent the end of a career — especially when they may be facing age discrimination and bias. Economics correspondent Paul Solman looks at the problem as part of his series Unfinished Business.
Tag Archives: Vaccine
Research:”The Costs Of A Covid-19 Vaccine” (Video)
As government and private money pour into the global race for a Covid-19 vaccine, drugmakers are under great pressure to keep the shot affordable while also keeping investors happy. WSJ explains what this means for the final price tag of the jabs.
Illustration: Crystal Tai
Health Update: The First Clinical Trial Of A “Coronavirus / Covid-19” Vaccine Begins (NIH)
From the National Institutes of Health (NIH) (March 17, 2020):
 “Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said NIAID Director Anthony S. Fauci, M.D. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said NIAID Director Anthony S. Fauci, M.D. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
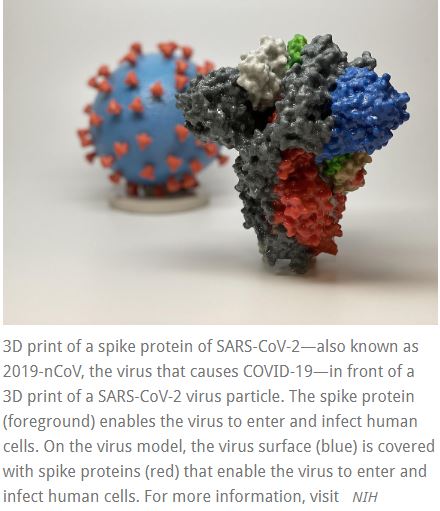
The vaccine is called mRNA-1273 and was developed by NIAID scientists and their collaborators at the biotechnology company Moderna, Inc., based in Cambridge, Massachusetts. The Coalition for Epidemic Preparedness Innovations (CEPI) supported the manufacturing of the vaccine candidate for the Phase 1 clinical trial.
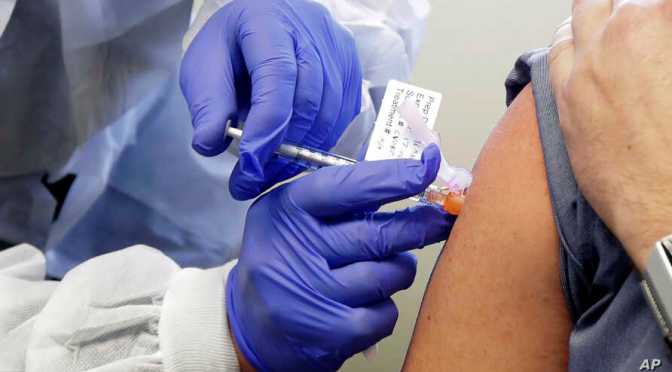
 A Phase 1 clinical trial evaluating an investigational vaccine designed to protect against coronavirus disease 2019 (COVID-19) has begun at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is funding the trial. KPWHRI is part of NIAID’s Infectious Diseases Clinical Research Consortium. The open-label trial will enroll 45 healthy adult volunteers ages 18 to 55 years over approximately 6 weeks. The first participant received the investigational vaccine today.
A Phase 1 clinical trial evaluating an investigational vaccine designed to protect against coronavirus disease 2019 (COVID-19) has begun at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is funding the trial. KPWHRI is part of NIAID’s Infectious Diseases Clinical Research Consortium. The open-label trial will enroll 45 healthy adult volunteers ages 18 to 55 years over approximately 6 weeks. The first participant received the investigational vaccine today.
The study is evaluating different doses of the experimental vaccine for safety and its ability to induce an immune response in participants. This is the first of multiple steps in the clinical trial process for evaluating the potential benefit of the vaccine.